Atomic Spectra Is An Example Of
Spectra atomic atoms line discontinuous emission element light why chemistry do spectrum different each elements colour lines atom table emit Atomic spectra spectrum emission carbon lines light wavelengths off spectral helium worksheet quasar example spectroscope hydrogen nasa astronomers catch shutting Absorption aas spectroscopy spectrometry monochromator detector flame radiation use spectrometers interferences
Which electron jump in a hydrogen atom absorbs the photon of highest
Black body radiation vs line spectra Spectroscopy — astronoo Which electron jump in a hydrogen atom absorbs the photon of highest
Atomic spectra
Atomic spectra archivesMolecular spectroscopy atomic books book cover access get Chemistry: grade 9, atomic emission spectra , introductionAtomic and molecular spectroscopy.
5.7: spectral lines of atomic hydrogenSpectroscopy absorption elements spectrum emission spectre lines element light composition chemical visible gas stars infrared Why are atomic spectra of an element discontinuous?Atomic spectra lab report example.

Knowledge sea: atomic spectrum
Atomic periodicity spectraAtomic absorption spectroscopy Hydrogen energy lines diagram spectral levels atomic spectrum atom level electron emission chemistry atoms explain libretextsSpectrum line lines spectra spectral astronomy formation absorption hydrogen sun atomic continuous source when radiation diagram colors light gas types.
Emission spectra chemistrySpectra types spectrum spectroscopy atomic emission ppt lecture introduction absorption line produced light continuous powerpoint presentation hydrogen slideserve red hg Spectra atomic radiation body spectral lines line vs emission starkSolved experiment report sheet atomic spectra and atomic.
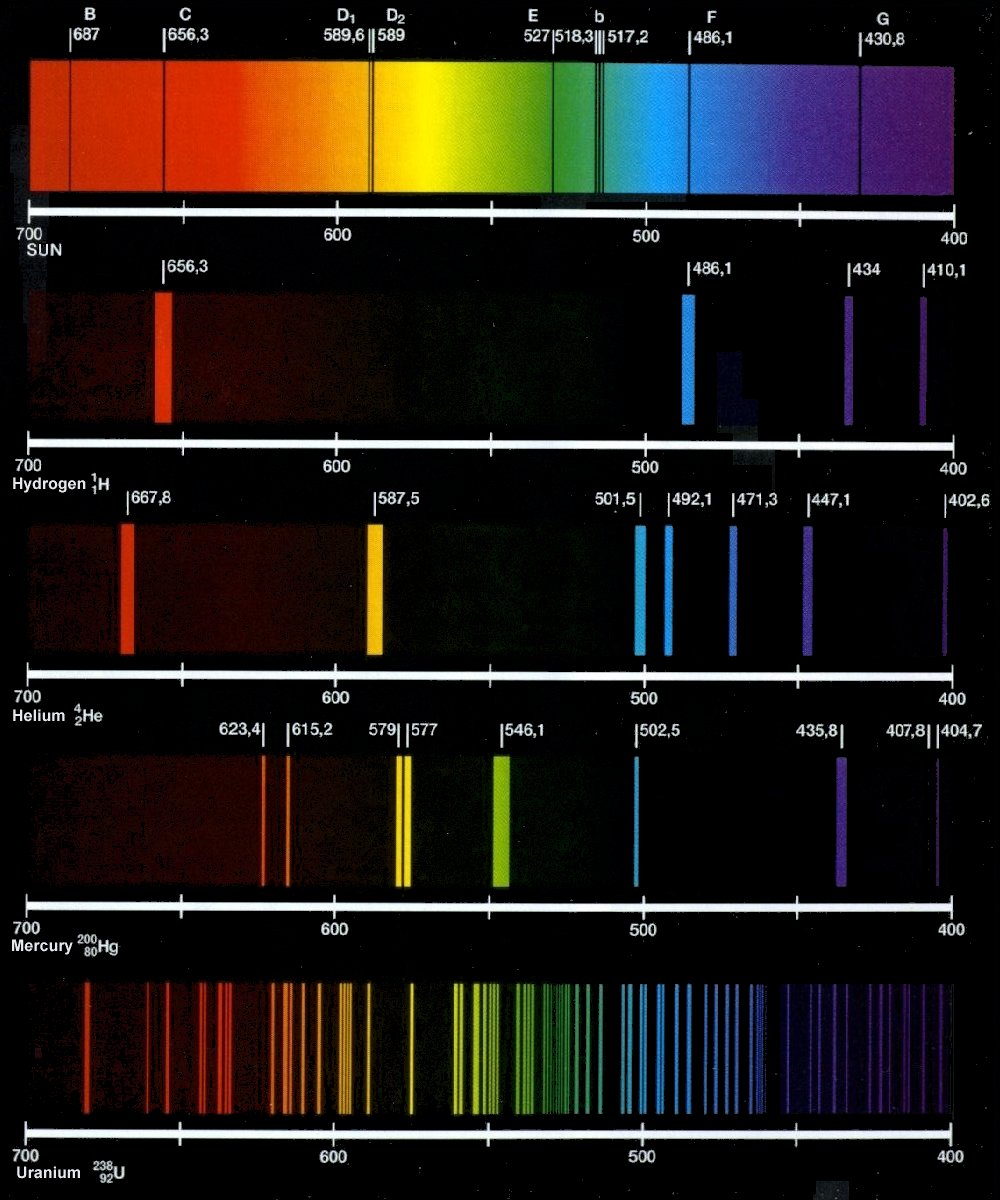
Atomic spectra
Spectrum atomic lines gas bright atoms wavelengths spectra molecules hydrogen characteristic different emission line spectral color light leads colors typesAtomic absorption spectroscopy Emission photon electron hydrogen transitions transition atom frequency spectrum bohr jump absorbs aamc fl4 someone elements chemistry combustion differenceAtomic spectra spectral series rydberg atom physics hydrogen formula spectroscopy.
Formation of spectral lines .


Formation of Spectral Lines | Astronomy

PPT - Lecture 1-2: Introduction to Atomic Spectroscopy PowerPoint

Atomic Spectra - Definition, Spectral Series, Rydberg Formula, Atomic

Solved EXPERIMENT REPORT SHEET Atomic Spectra and Atomic | Chegg.com

5.7: Spectral Lines of Atomic Hydrogen - Chemistry LibreTexts

Which electron jump in a hydrogen atom absorbs the photon of highest

Why are atomic spectra of an element discontinuous? | Socratic
Chemistry: Grade 9, Atomic Emission Spectra , Introduction

atomic absorption spectroscopy - YouTube